In Vitro Testing Templates
In Vitro Testing: Accreditation and Certifications for Testing with Radioactive Material
Welcome to our comprehensive guide on in vitro testing, also known as testing with radioactive material. This alternate name refers to a specific set of documents and certifications related to conducting tests in controlled laboratory settings. Whether you're a researcher, a laboratory technician, or an organization looking to comply with regulatory requirements, understanding the process and obtaining the necessary accreditation is vital.
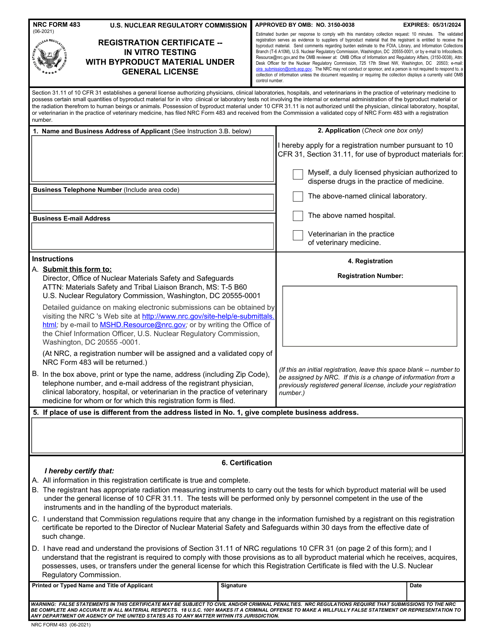
At our website, we provide a wealth of information and resources to help you navigate the complex world of in vitro testing. Our dedicated team has compiled an extensive collection of documents, including registration certificates and various forms required for testing with radioactive material under general license.
From the Registration Certificate in Vitro Testing with Radioactive Material issued in Georgia (United States) to the Form DWMRC-07 Registration Form for in Vitro Testing with Radioactive Material under General License in Utah, our database encompasses a wide range of regulatory documents from different states. We also offer the Form SFN8423 (RCP-18) Certificate for in Vitro Testing with Radioactive Material under General License in North Dakota, as well as the Form HEA5518 In-vitro Testing with Radioactive Material Form in Ohio.
Our mission is to streamline the accreditation process and ensure that you have access to all the necessary documentation. By centralizing these resources, we aim to save you time and effort when dealing with regulatory compliance.
The importance of in vitro testing cannot be overstated. This form of testing plays a crucial role in various fields, including pharmaceuticals, biotechnology, and medical research. It allows scientists and researchers to conduct experiments and analyze the effects of substances on biological systems, without exposing humans or animals to potential risks.
By providing comprehensive guidance and access to the required paperwork, we help professionals in the field achieve compliance with the relevant regulations governing in vitro testing with radioactive material.
In summary, if you are looking for information, forms, and certificates related to in vitro testing, you have come to the right place. Our website offers a vast collection of documents, making it easier for you to obtain the necessary accreditation. Save time and ensure compliance by utilizing our resources. Start exploring our database today and take your in vitro testing to new heights.
Documents:
13
This form is used for certificate in vitro testing with radioactive material under general license in Minnesota.
This document is for obtaining a registration certificate for conducting in vitro testing with radioactive material under a general license in Georgia, United States.
This Form is used to apply for a certificate for conducting in vitro testing activities involving radioactive material under general license in Maryland.
This form is used for registering and obtaining a certificate for in-vitro testing that involves the use of radioactive material under a general license in Rhode Island.
This form is used for registering in vitro testing with radioactive material under a general license in the state of Utah.
This form is used for obtaining a certificate for conducting in-vitro testing using radioactive material under a general license in North Dakota.
This Form is used for obtaining a certificate for conducting in-vitro testing with radioactive material under a general license in North Dakota.
This form is used for in-vitro testing with radioactive material in the state of Ohio.
This form is used for obtaining a certificate for conducting in vitro testing with radioactive material under general license in the state of Florida.