Canadian Federal Legal Forms and Templates
Documents:
5112
This document is used for requesting payment for completed works in Canada.
This form is used for requesting approval for special purchases and services in Canada.
Forme AMC-GAC2655F Entente De Formation - Stagiaire Ou Membre De Mission Technique - Canada (French)
This document is a training agreement form for interns or members of technical missions in Canada. It is written in French.
This document is a form used in Canada to apply for the allocation of a tariff quota for the importation of beef and veal. The form is specifically for the period from January 1st to December 31st, 2019.
This Form is used for requesting additional import authorization and local supplies for turkey and turkey products in Canada. (French language)
This document is a French form used in Canada to request an authentication service.
This type of document is used in Canada for applying for a brokerage license and related firearm details.
This document is used for requesting a brokerage license in Canada. It contains general information required for the application.
This form is used for applying for a brokerage license in Canada. It gathers information about controlled goods.
This type of document is used for declaring outstanding amounts owed to Her Majesty - Canada under the MAECD-DFATD2555F form.
This document is a form used in Canada for suppliers to apply for electronic payment methods.
This document is a form for attesting the labels and packaging of over-the-counter medications sold in Canada.
This type of document is used for certification of presentations or applications for human drugs and/or disinfectants in Canada.
Aucune
This Form is used for listing and regulating patented medications in Canada. It is used to fill out a form for each patent by DIN.
This document is used for over-the-counter medications for listed diseases in Schedule A, excluding natural health products - Canada.
This document is used for certifying the translation of a product monograph in Canada.
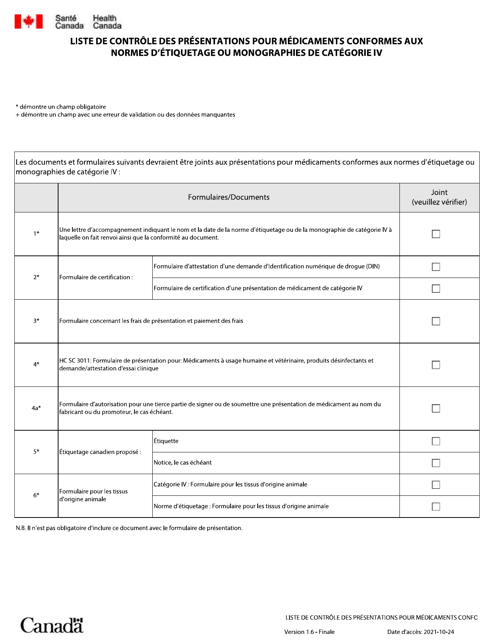
This document is for pharmaceutical companies in Canada, providing them with a checklist for submissions of medications that comply with labeling standards or Category IV monographs. It is aimed at ensuring that regulatory compliance is met for drug labeling and presentation. It is provided in French.
This document is a template used for assessing the safety and efficacy of protocols in clinical trial applications in Canada. It helps ensure that the study follows proper guidelines and regulations to protect participants and gather reliable data.
This document is an safety assessment update for a sponsor in Canada. It includes an attestation from the sponsor.
This document is for the attestation of the promoter of generic drugs in Canada. It provides updates to the product monographs to ensure compliance with the PRC regulations.
This form is used for requesting fee remission and attesting the right to sell drugs in Canada.
This Form is used for requesting the emergency release of drugs in Canada.
This form is used for applying for a Veterinary Drug Experimental Studies Certificate (ESC) in Canada.
This form is used for applying for long-term disability insurance under the Public Service Management Insurance Plan in Canada. It is available in both English and French.
This form is used for submitting payment transactions in Canada. It is available in both English and French.
This Form is used for the Public Service Management Insurance Plan in Canada. It is available in both English and French languages.
This form is used for Long-Term Disability Insurance under the Public Service Management Insurance Plan in Canada. It is available in both English and French.
This Form is used for enrolling in a plan and acknowledging membership in Canada. It is available in English and French.
This form is used for applying for relief provisions in the Public Service Health Care Plan (PSHCP) in Canada.
This form is used for interpreting the results of a medical examination for pension purposes in Canada. It is available in both English and French.
Form PWGSC-TPSGC2002 Declaration of Attendance at an Educational Institute - Canada (English/French)
This form is used for declaring your attendance at an educational institute in Canada. It is available in both English and French.
This Form is used for changing the name or beneficiary of the post-retirement life insurance plan in Canada. The form is available in both English and French.
This form is used by participants in the Supplementary Death Benefit (SDB) Plan in Canada who wish to continue their participation. It is available in both English and French.