Canadian Federal Legal Forms and Templates
Documents:
5112
This form is used for applying for a share of the Eggs and Egg Products Tariff Rate Quota (TRQ) in Canada. It is available in both English and French.
This form is used for ordering Quota or Permit Utilization Reports in Canada. The form is available in both English and French languages.
This document is used for applying for a share of the Chicken Tariff Rate Quota (TRQ) in Canada. The application can be submitted in English or French.
This form is used for applying for a share of the Turkey Tariff Rate Quota (TRQ) in Canada. It is available in both English and French.
This form is used for food service applicants in Canada to provide a summary sheet of information. It is available in both English and French.
This Form is used for applying to receive a portion of the Yogurt Trq in Canada. The form is available in both English and French.
This form is used for Canadian refined sugar allocation holders to apply for a share of the sugar export quota. It is available in both English and French.
This form is used for collecting information about clinical trial sites in Canada. It is available in both English and French.
This document is a submission certification for the approval of human and disinfectant drugs in Canada. It is used to ensure the safety and effectiveness of these drugs before they can be marketed and sold to the public.
This form is used by allocation holders of sugar-containing products in Canada to retain their share of the Sugar-Containing Products TRQ. It is available in both English and French.
This form is used for requesting administrative changes in certification for human and/or disinfectant drug submissions and applications in Canada.
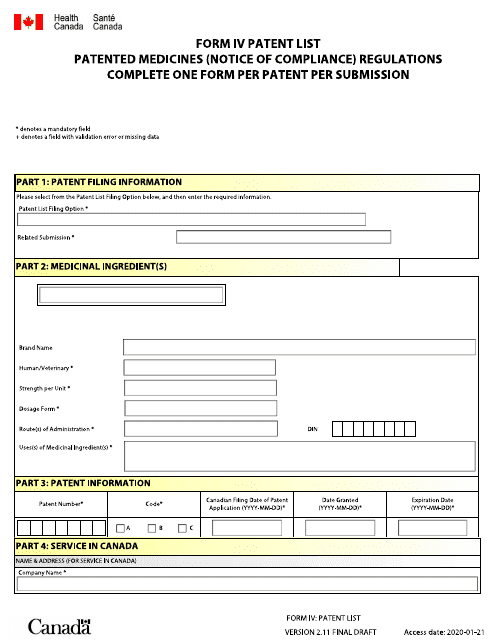
This form is used for listing patented medicines under the Patent List in accordance with the Notice of Compliance Regulations in Canada. It is available in both English and French.
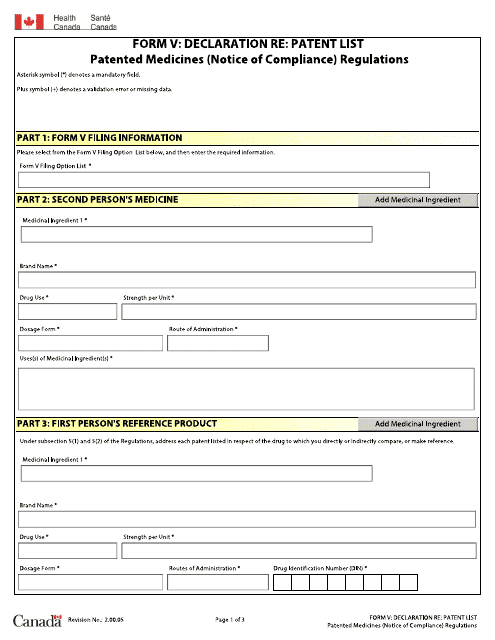
This form is used for declaration pertaining to the Patent List of patented medicines under the Notice of Compliance Regulations in Canada.
This form is used for applying for a Drug Master File (DMF) in Canada. The form is available in both English and French.
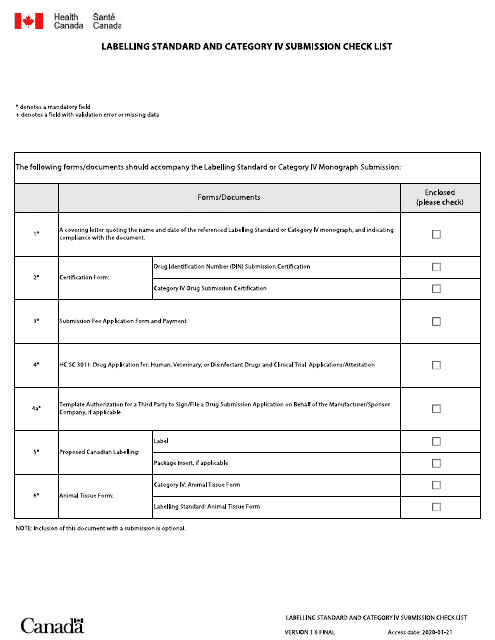
This document is a checklist used in Canada for labeling products. It includes requirements for both English and French labeling and is used for Category IV submissions.
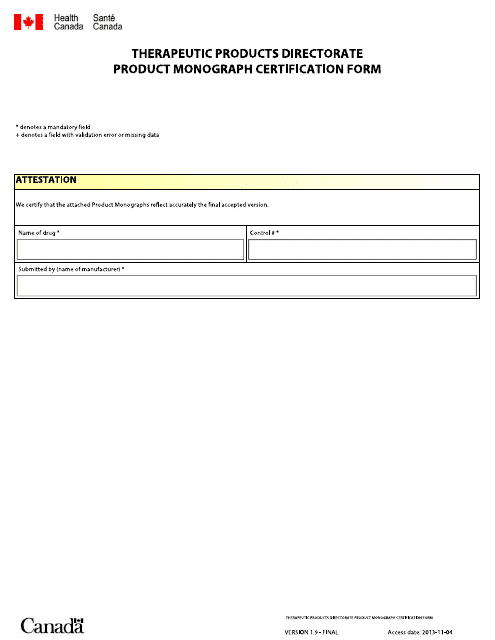
This Form is used for certifying the product monograph in Canada. It is available in both English and French languages.
This form is used for certifying the translation of a product monograph (a document containing information about a pharmaceutical product) from English to French or vice versa in Canada. It is required to ensure accurate and reliable translation for regulatory purposes.
This document is a certificate issued by Health Canada for new drug submissions and related supplements and changes. It confirms the acceptance and review status of a drug application in Canada.
This form is used for attesting to the details of a non-prescription drug monograph in Canada.
This document is used for reporting nonprescription products (excluding natural health products) in Canada. It is available in both English and French.
This form is used for applying for various services at the Indian Consulate in Canada.
This form is used for the checklist of documents submitted by the appellant to the Refugee Appeal Division of the Immigration and Refugee Board of Canada.
This form is used for identifying potential security risks in IRB proceedings in Canada.
This form is used for notifying the withdrawal of an appeal in Canada.
This Form is used for notifying the Canadian government that a representative is providing their services without charging a fee or receiving any other form of compensation. The form is available in both English and French.
This form is used for applying for an extension of time to respond to an appeal in Canada.
This form is used for expressing the intention to proceed with a certain action or process in Canada.
This document is used for appointing a designated representative for accompanied minors in Canada.
This Form is used for applying to change the location of a legal proceeding in Canada.
This form is used for obtaining a medical certificate in Canada.
This form is used for listing the documents belonging to a Minister in Canada. It helps organize and keep track of the documents handled by the Minister.
This Form is used for applying for an extension of time to file or perfect an appeal in Canada.
This form is used for filing a Notice of Appeal in Canada to appeal a Removal Order.
This form is used for providing the information of a bondsperson in Canada.