Fill and Sign United States Federal Legal Forms
Documents:
24261
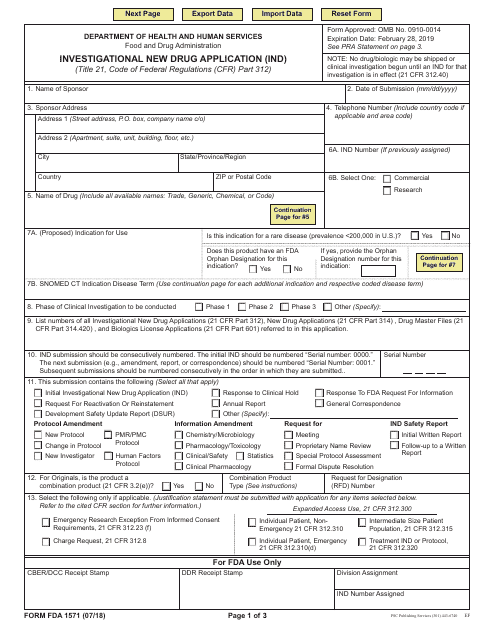
This type of document, FDA Form 1571, is used for submitting an Investigational New Drug Application (IND). It is required by the U.S. Food and Drug Administration (FDA) when seeking approval to conduct clinical trials of a new drug.
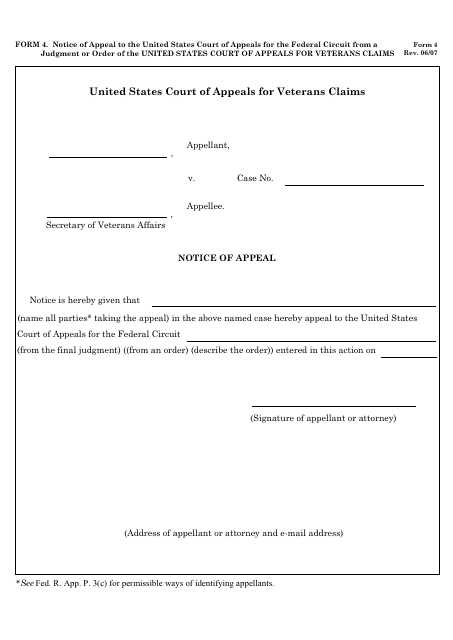
This form is used for filing a notice of appeal to the United States Court of Appeals for the Federal Circuit. It is specifically for cases where there has been a judgment or order issued by the United States Court of Appeals for Veterans Claims.
This Form is used for applying to represent a client in the United States Court of Appeals for Veterans Claims as an out-of-state attorney.
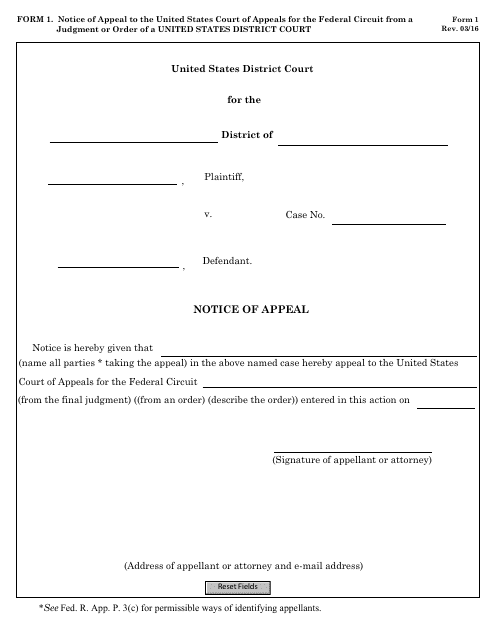
This Form is used for filing an appeal to the United States Court of Appeals for the Federal Circuit from a judgment or order of a United States District Court.
This document is a template for a brief cover used in district court cases. It provides a concise summary of the case and is typically filed by the plaintiff or defendant.
This form is used for certifying compliance with Rule 32(A) of a certain regulation.
This Form is used for entering appearance in a legal proceeding. It is used to formally notify the court and other parties involved that an attorney is representing a party in the case.
This document is for declaring secure tests and test items before they are used in a secure test.
This Form is used for certifying plug and ring gauges used in the Naval Sea Systems Command (NAVSEA) 9243/1. It validates the accuracy and suitability of these gauges for use in various applications.
This Form is used for certifying the major sub assembly of a propeller and propulsor.
This form is used for visual inspection of plug and ring gages.
This Form is used for propulsor installation and certification in naval vessels. It ensures proper installation of propulsor systems and verifies their operational readiness.
This form is used for certifying the propeller blade gage for the NAVSEA9245/14 model.