Health Canada Forms
Health Canada is the federal department responsible for promoting and protecting the health and well-being of Canadians. It plays a crucial role in ensuring that Canadians have access to safe and effective health products, such as drugs, medical devices, and natural health products. Health Canada also sets guidelines and regulations for food safety, environmental health, and the safety of consumer products. Additionally, Health Canada provides information and resources to help Canadians make informed decisions about their health and safety.
Documents:
79
This form is used for requesting reimbursement for medical transportation expenses in the Atlantic region of Canada.
This form is used for clients of the Non-Insured Health Benefits (NIHB) program in Canada to request reimbursement for eligible expenses.
This form is used for applying for a share of the Products of Natural Milk Constituents Tariff Rate Quota (TRQ) in Canada. The form is available in both English and French.
This form is used for collecting information about clinical trial sites in Canada. It is available in both English and French.
This document is a submission certification for the approval of human and disinfectant drugs in Canada. It is used to ensure the safety and effectiveness of these drugs before they can be marketed and sold to the public.
This form is used for requesting administrative changes in certification for human and/or disinfectant drug submissions and applications in Canada.
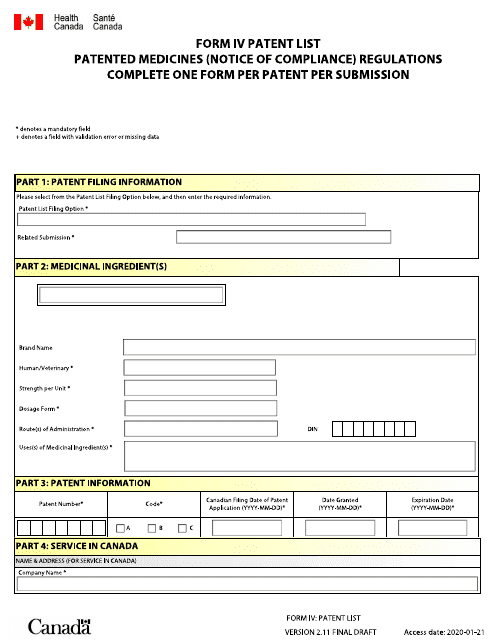
This form is used for listing patented medicines under the Patent List in accordance with the Notice of Compliance Regulations in Canada. It is available in both English and French.
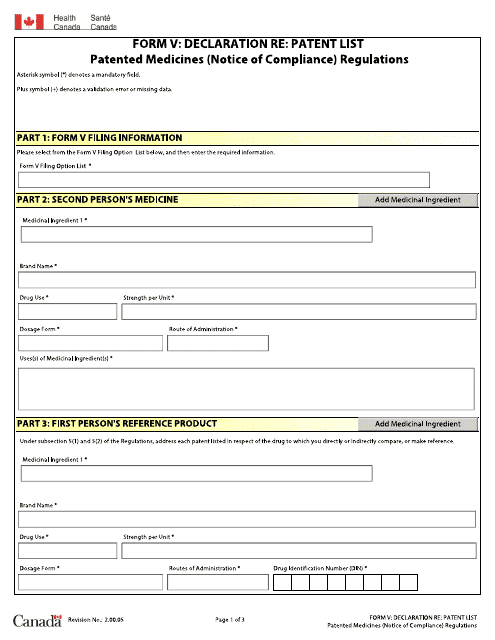
This form is used for declaration pertaining to the Patent List of patented medicines under the Notice of Compliance Regulations in Canada.
This form is used for applying for a Drug Master File (DMF) in Canada. The form is available in both English and French.
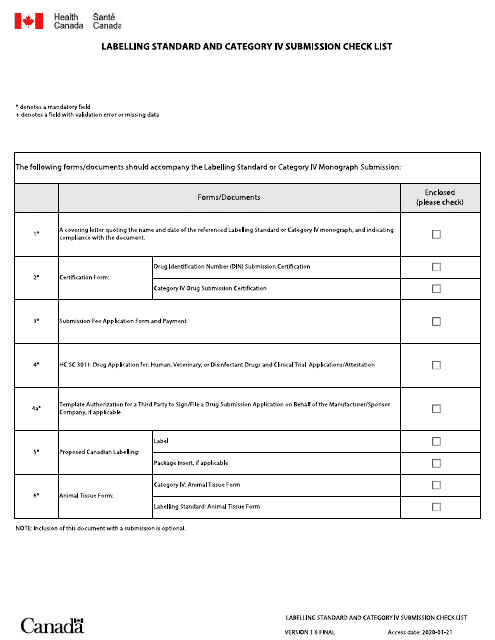
This document is a checklist used in Canada for labeling products. It includes requirements for both English and French labeling and is used for Category IV submissions.
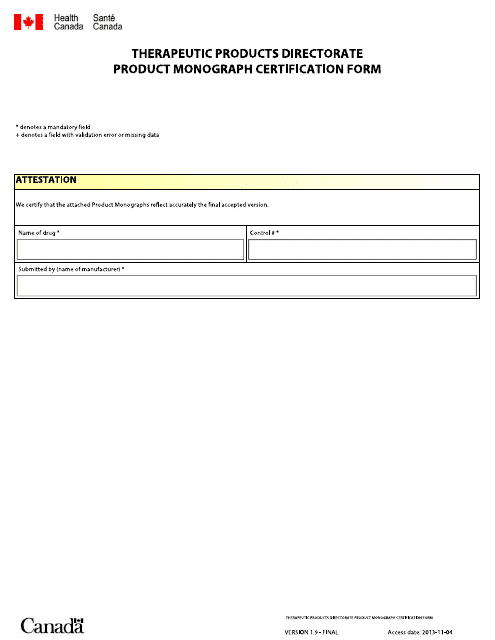
This Form is used for certifying the product monograph in Canada. It is available in both English and French languages.
This form is used for certifying the translation of a product monograph (a document containing information about a pharmaceutical product) from English to French or vice versa in Canada. It is required to ensure accurate and reliable translation for regulatory purposes.
This document is a certificate issued by Health Canada for new drug submissions and related supplements and changes. It confirms the acceptance and review status of a drug application in Canada.
This form is used for attesting to the details of a non-prescription drug monograph in Canada.
This document is used for reporting nonprescription products (excluding natural health products) in Canada. It is available in both English and French.
This form is used for reporting serious adverse drug reactions that occur in hospitals in Canada.
This form is used for applying to act as a single health information custodian in Canada. It provides instructions on how to complete the application.
This document provides detailed information about the advance payment requirements for master files related to human and disinfectant drugs, as well as certificate of supplementary protection applications in Canada.
This document provides information on how to make payment for an invoice in Canada. It includes details such as payment methods, deadlines, and any additional instructions for settling the invoice amount.
This document is used for submitting an application fee for human and disinfectant drugs in Canada.
This document is used for notifying compliance changes at Level III in Canada.
This type of document is used for product monograph updates related to generic drugs in Canada to be in line with the Common Review Process (CRP).
This document is a certificate that is issued in Canada for a new drug submission, supplement to a new drug submission, supplement to an abbreviated new drug submission, abbreviated new drug submission, or notifiable change. It is used to officially acknowledge the submission of these types of documents related to drugs in Canada.
This document is a sponsor attestation checklist for abbreviated new drug submissions (ANDSS) in Canada. It provides a list of requirements and criteria that sponsors must meet when submitting their abbreviated new drug applications.
This Form is used for the certification of labels and packages for prescription products in Canada.
This Form is used for submitting an application fee for human and disinfectant drugs in Canada.
This Form is used for paying the application fee for the Master File (Mf) for Human Drugs in Canada.
This Form is used for submitting a certificate for a new drug submission, supplement to a new drug submission, supplement to an abbreviated new drug submission, abbreviated new drug submission, or notifiable change in Canada.