Controlled Substance Templates
Are you in need of information regarding controlled substances? Look no further, as we have a comprehensive collection of documents related to substance control. These documents provide critical information and resources for individuals and organizations dealing with controlled substances.
Our extensive library includes various forms and templates such as the Form CR-251 Physician and Pharmacy Conditions for Maine, Form MO580-3009 Dental Application for a Controlled Substances Registration and Practitioner Availability Census for Missouri, and Form MCS-1 (EFO00112) Controlled Substance Tax Stamp Order for Idaho, among others. These documents cover a wide range of topics, ensuring that you have access to the information you need promptly.
Whether you are a healthcare professional, pharmacist, or simply looking for general information on controlled substances, our collection has you covered. Our documents not only provide guidance on the registration processes and requirements but also cover relevant legislation and policies surrounding controlled substances.
By utilizing our controlled substance document database, you can ensure compliance with state and federal regulations while staying informed about the latest developments in this field. We understand that navigating through the complex world of substance control can be challenging, which is why our documents are designed to make the process more accessible and straightforward.
Browse our controlled substance forms and templates and access the information you need today. Stay informed and compliant with our comprehensive collection of controlled substance documents.
Documents:
120
This document is used for tracking the return of Fentanyl patches to pharmacies.
This form is used for keeping track of the inventory and distribution of narcotics and controlled substances at Isse Pharmacy Services.
This form is used for applying for the authority to dispense controlled substances or dangerous drugs or both in the state of Nevada.
This form is used for conducting a biennial inventory of controlled substances at Yale University's Environmental Health & Safety department.
This document is used for keeping track of controlled substances and their inventory records.
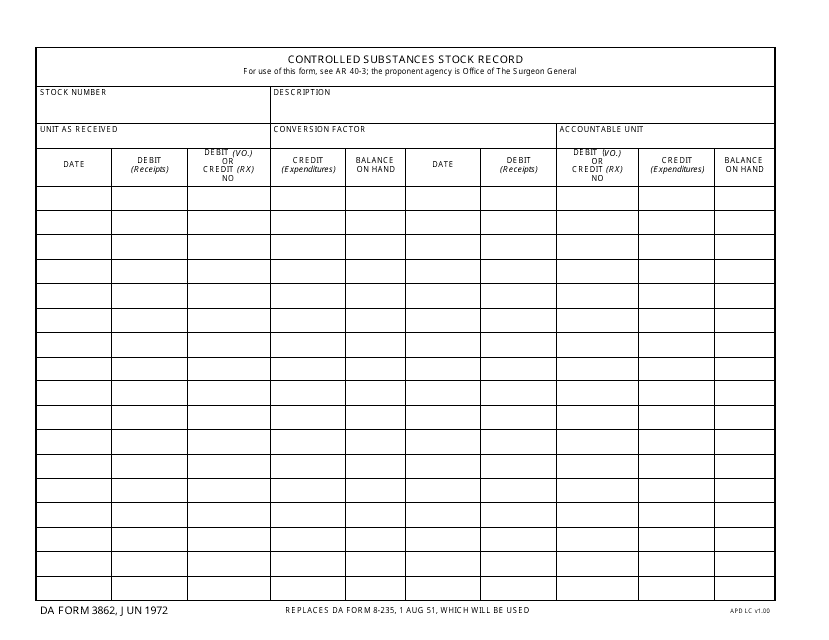
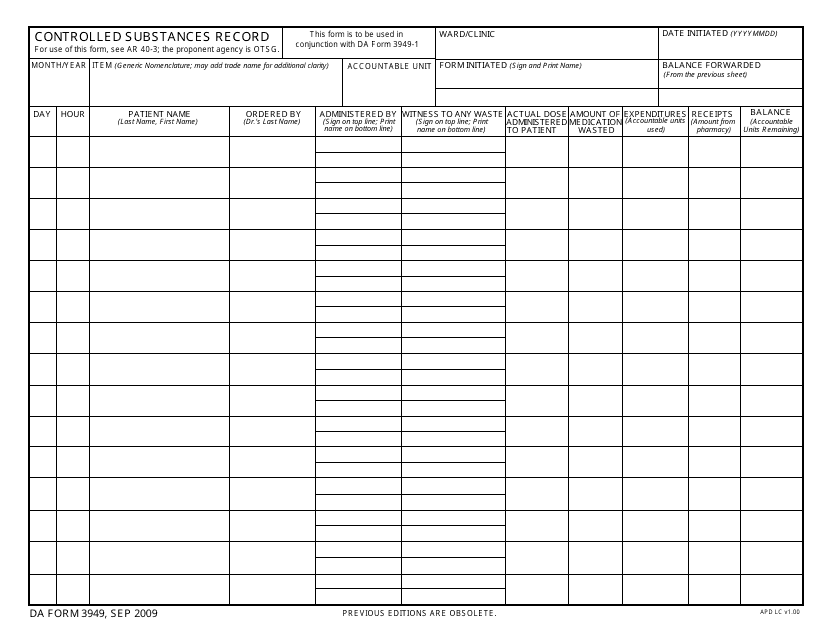
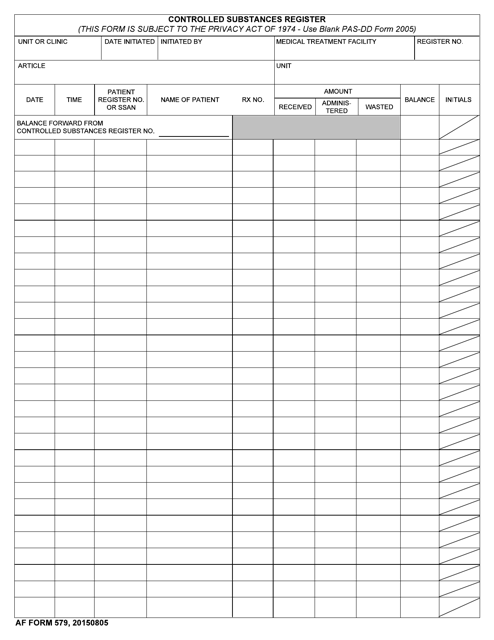
This type of document, DA Form 3949 Controlled Substances Record, is used for tracking and documenting the use and distribution of controlled substances within the military.
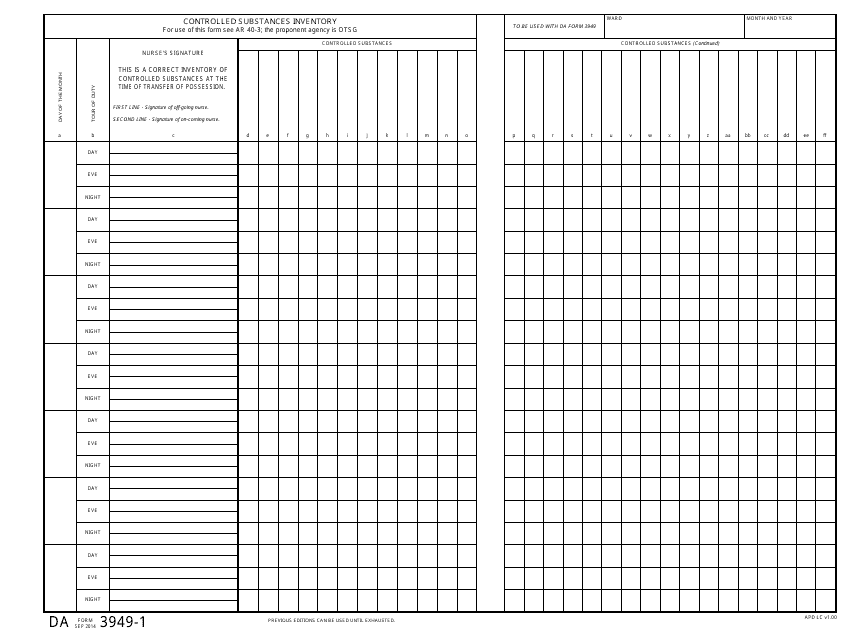
This document is used for conducting an inventory of controlled substances. It is used by the military to ensure accountability and proper management of these substances.
This Form is used for applying for an import quota for Ephedrine, Pseudoephedrine, and Phenylpropanolamine with the DEA.
This document is used to apply for a procurement quota with the Drug Enforcement Administration (DEA). It is required for individuals or businesses that want to procure controlled substances for legitimate purposes.
This Form is used to apply for a permit to export controlled substances.
This Form is used for applying for a permit to import controlled substances for domestic and/or scientific purposes from the DEA (Drug Enforcement Administration).
This Form is used for tracking and recording the use of controlled substances for nonhuman purposes.
This form is used to notify and document the transfer of controlled substances within an institute.
This form is used for requesting controlled substances for nonhuman use.
This Form is used for veterinarians in Michigan to prescribe certain medicated feed for animals. It helps ensure the safe and proper use of medicated feed to promote animal health.
This Form is used for conducting an annual inventory of controlled substances in the state of Missouri.
This form is used for reporting the loss or theft of controlled substances in the state of Missouri.
This Form is used for transferring controlled substances in the state of Missouri. It helps ensure proper documentation and accountability for the movement of these substances.
This document is used for keeping track of controlled substances in Missouri. It helps monitor the inventory and ensure compliance with laws and regulations.
This form is used for granting authorization to dispense medication in the state of Georgia, United States.
This form is used for applying to conduct a pharmaceutical collection event for controlled substances in New York.
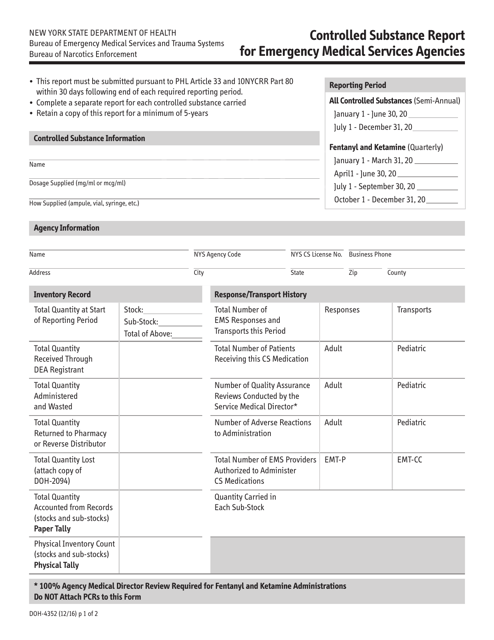
This form is used for emergency medical services agencies in New York to report controlled substances.
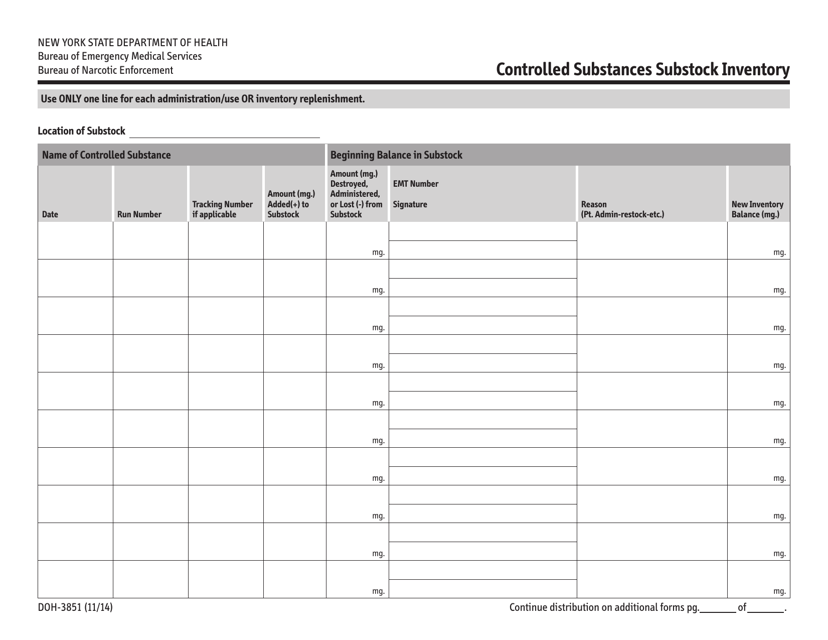
This document is a form used for inventory management of controlled substances in New York. It helps organizations keep track of their substock inventory.
This form is used for verifying the usage of controlled substances in New York. It is important for monitoring and ensuring compliance with regulations.
This form is used for inmates in Oklahoma to provide consent for pain treatment with controlled substances.
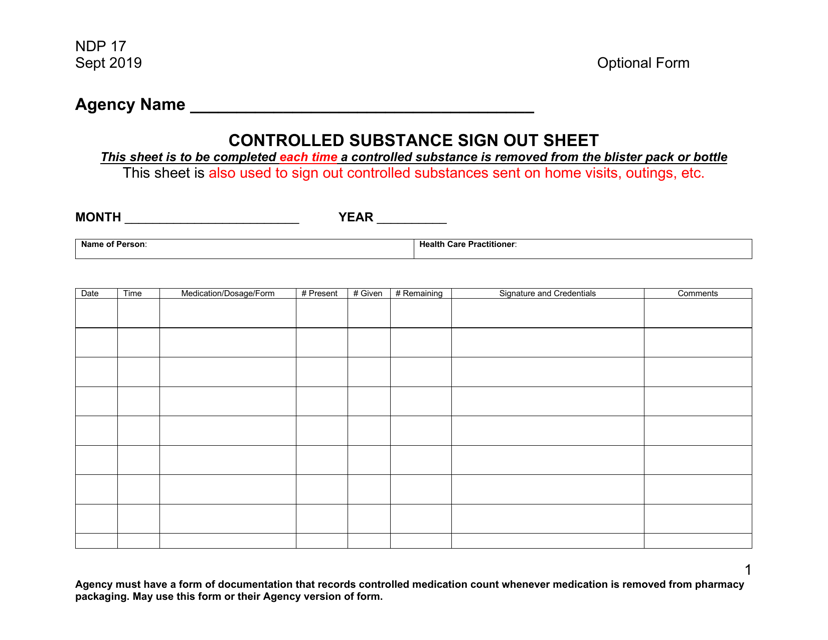
This form is used for keeping track of controlled substances in a secure and controlled manner. It helps maintain accurate records of the quantities and usage of controlled substances.
This document is used for making adjustments to the inventory of controlled substances in the military.
This form is used for providing a statement for compound prescription in the state of Washington. It is required for compounds that are custom-made medications.
This form is used for requesting authorization for the use of buprenorphine transdermal patches in the state of Washington.
This Form is used for registering under the Rhode Island Uniform Controlled Substances Act.
This document is used for applying for a retail pharmacy license and controlled substances registration in Rhode Island. The form is necessary for pharmacies to legally operate and handle controlled substances.
This form is used for tracking and documenting controlled substances in New York. It helps ensure proper handling and accountability of these substances.
This document is used for obtaining informed consent from patients in Hawaii who are prescribed opioid pills. It outlines the potential risks, benefits, and alternatives to opioid medications.
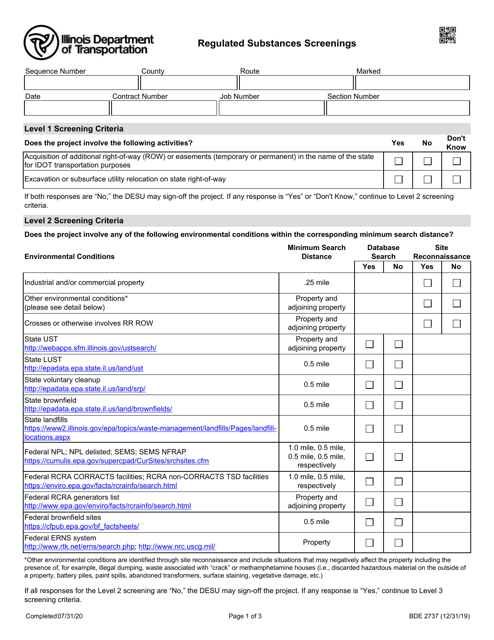
This form is used for regulated substances screenings in the state of Illinois. It helps ensure compliance with regulations regarding the use of certain substances.
This form is used for requesting prior authorization for high dose opioid drugs in the state of Minnesota.
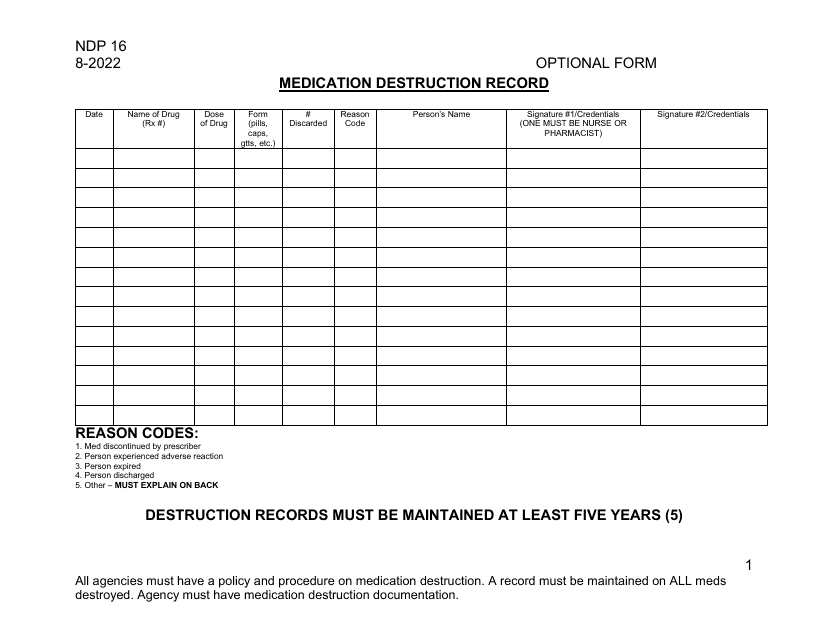
This form is used to request approval for the disposal or destruction of controlled substances in New York.