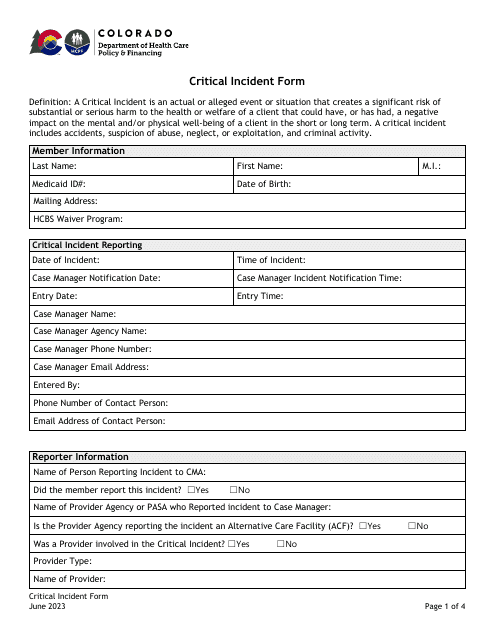
Adverse Drug Reaction Templates
Adverse Drug Reaction - Safeguarding Medication Safety
Ensure the safety and well-being of both human and animal patients with our comprehensive collection of documents on adverse drug reactions. Also known as adverse drug events or ADRs, adverse drug reactions refer to harmful, unintended, and potentially dangerous effects that occur due to the use of medications or drugs.
Our extensive range of documents, including the Form FDA1932 Veterinary Adverse Drug Reaction, Lack of Effectiveness, Product Defect Report and the Form DCF-465B Suspected Adverse Drug Reaction Reporting Form - Connecticut, provide a valuable resource for healthcare professionals, veterinarians, and regulatory agencies to report, track, and analyze adverse drug reactions.
Stay informed and contribute to improving medication safety by utilizing our collection of documents on adverse drug reactions. Together, we can enhance pharmacovigilance, identify potential risks, and develop strategies to minimize harm caused by medications.
Documents:
8
This form is used for reporting suspected adverse drug reactions in the state of Connecticut.
This Form is used for reporting suspected adverse drug reactions in Oklahoma.