Drug Development Templates
Are you looking to bring a new pharmaceutical product to market? Our drug development services can help you navigate the complex process of obtaining regulatory approval and ensuring the safety and effectiveness of your product.
Our team of experienced professionals understands the intricacies of the drug development process and can provide expert guidance every step of the way. From submitting the necessary forms, such as the FDA356h Application to Market a New or Abbreviated New Drug or Biologic for Human Use, to preparing and submitting the Form FDA1571 Investigational New Drug Application (IND), we have the knowledge and expertise to streamline the process and maximize your chances of success.
Additionally, we can assist with formal meetings between you, as the sponsor, and the FDA, as outlined in the guidance for industry document "Formal Meetings Between the FDA and Sponsors or Applicants of PDUFA Products." These meetings are a crucial opportunity to discuss your drug development plans, address any concerns, and ensure that all regulatory requirements are met.
For those seeking approval in Canada, we also provide support for the Drug Master File (DMF) application process. The DMF serves as a comprehensive document containing detailed information about the manufacturing, processing, and packaging of your pharmaceutical product.
Our drug development services are designed to streamline the regulatory approval process and help you bring your pharmaceutical product to market as efficiently and safely as possible. Trust our team of experts to navigate the complexities of drug development, so you can focus on what you do best – improving patients' lives.
Documents:
9
This document is a form used for requesting tests from Avista Pharma Solutions.
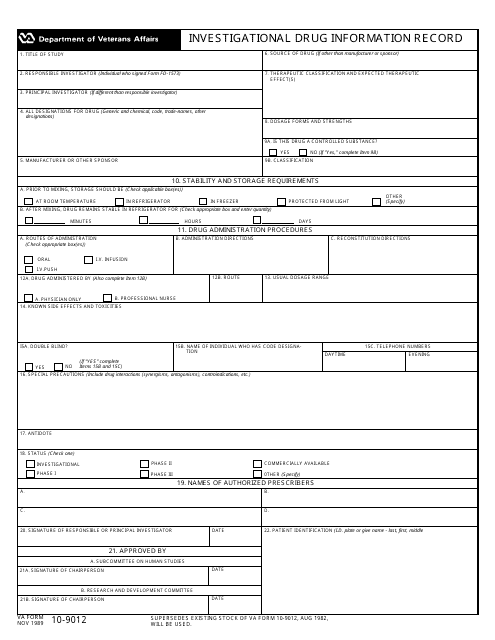
This document is used for recording information about investigational drugs.
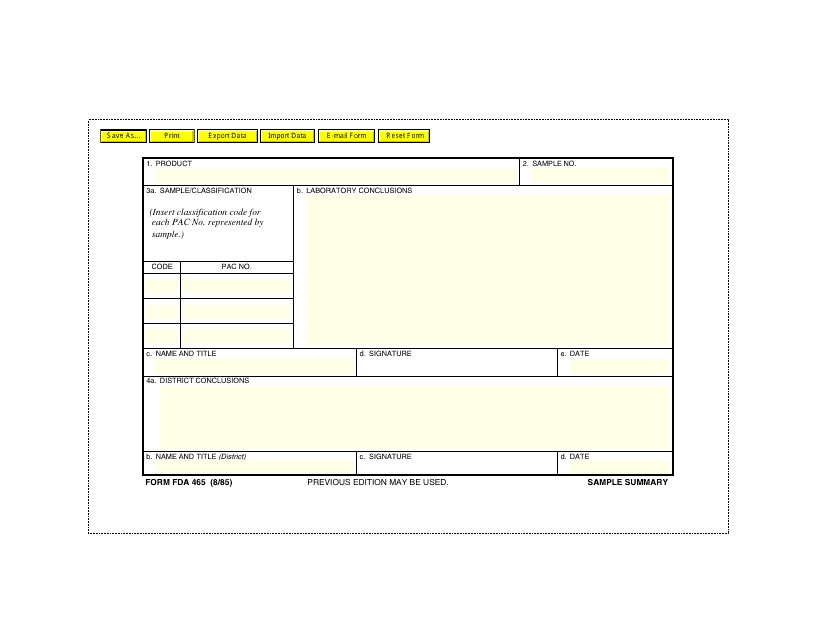
This form is used for summarizing the samples submitted to the U.S. Food and Drug Administration (FDA). It helps track and document the information related to the samples, including their source, testing methods, and results.
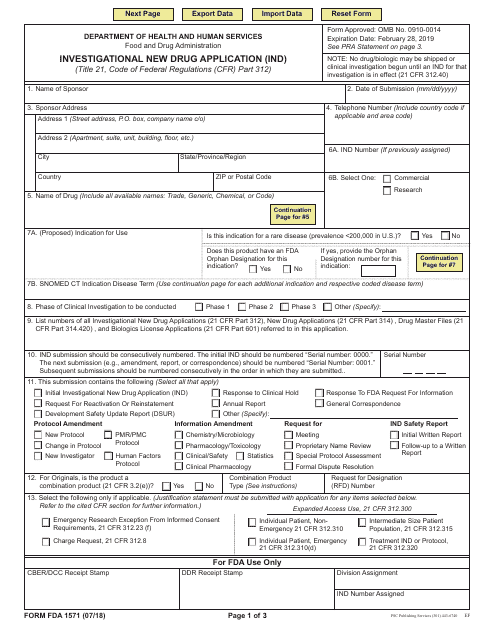
This Form is used for submitting an Investigational New Drug Application (IND) to the FDA. It provides instructions on how to complete the application for conducting clinical trials of a new drug.
This type of document, FDA Form 1571, is used for submitting an Investigational New Drug Application (IND). It is required by the U.S. Food and Drug Administration (FDA) when seeking approval to conduct clinical trials of a new drug.
This document is a certificate that is issued in Canada for a new drug submission, supplement to a new drug submission, supplement to an abbreviated new drug submission, abbreviated new drug submission, or notifiable change. It is used to officially acknowledge the submission of these types of documents related to drugs in Canada.
Formal Meetings Between the FDA and Sponsors or Applicants of Pdufa Products - Guidance for Industry
This document is for applying for a Drug Master File (DMF) in Canada. It is used to provide information about the manufacturing, processing, and formulation of a drug product.